Atomic and Nuclear Physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and the processes by which these arrangements change. This includes ions as well as neutral atoms and, unless otherwise stated, for the purposes of this discussion it should be assumed that the term atom includes ions. Atomic physics also helps to understand the physics of molecules, but there is also molecular physics, which describes the physical properties of molecules.
Nuclear physics is the field of physics that studies the constituents(protons and neutrons) and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation, but modern nuclear physics contains also particle physics, which is taught in close association with nuclear physics. The nuclear physics has provided application in many fields, including those in nuclear medicine and magnetic resonance imaging, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology.
The radius of the Nucleus
‘R’ represents the radius of the nucleus.
- Ro is the proportionality constant
- A is the mass number of the element
Total number of protons & neutrons in a nucleus
The mass number (A), also known as the nucleon number, is the total number of neutrons and protons in a nucleus.
| A = Z + N |
Mass Defect
Mass defect takes place when some of the mass is lost during the nuclei generation.
Where, M is the mass of the nucleus, is the difference between the mass of the nucleons and mass of the nucleus, is the Mass of the Proton and is the mass of the Neutron.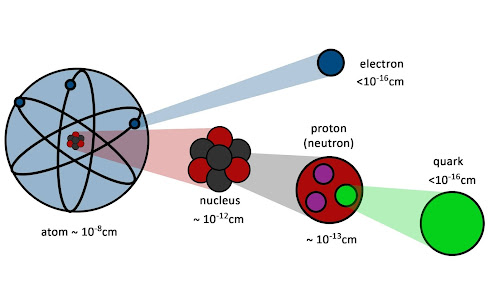

Chain reaction:- A nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The “one or more” is the key parameter of reactor physics. To raise or lower the power, the number of reactions must be changed (using the control rods) so that the number of neutrons present (and hence the rate of power generation) is either reduced or increased.
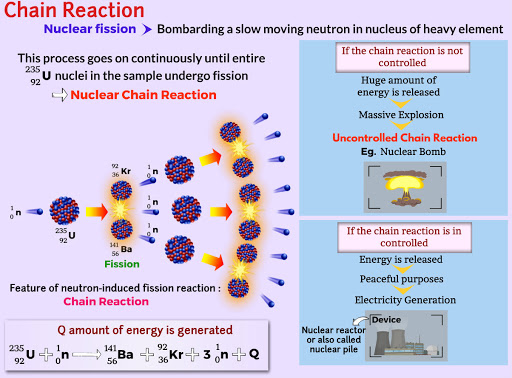
A nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions.
Mass and Energy:- Nuclear energy comes either from spontaneous nuclei conversions or induced nuclei conversions. Among these conversions (nuclear reactions) belong for example nuclear fission, nuclear decay and nuclear fusion. Conversions are associated with mass and energy changes. One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible, one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula:

where M is the small amount of mass and C is the speed of light. What that mean? If nuclear energy is generated (splitting atoms, nuclear fusion), a small amount of mass transforms into pure energy (such as kinetic energy, thermal energy, or radiant energy).
Nuclear Stability:- It is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This graph shows a plot of the referred to nuclides as a component of their nuclear and neutron numbers. It very well may be seen from the outline that there are a greater number of neutrons than protons in nuclides with Z more noteworthy than around 20 (Calcium). These additional neutrons are essential for the soundness of the heavier chores. The overabundance neutrons act to some degree like atomic paste.
Nuclear Stability Chart of Uranium
Nuclear reaction:- A nuclear reaction is considered to be the process in which two nuclear particles (two nuclei or a nucleus and a nucleon) interact to produce two or more nuclear particles or ˠ-rays (gamma rays). Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. Now and then if a core connects with another core or molecule without changing the idea of any nuclide, the procedure is alluded to an atomic dispersing, instead of an atomic response. Maybe the most outstanding atomic responses are the atomic combination responses of light components that power the vitality creation of stars and the Sun. Regular atomic responses happen additionally in the association between infinite beams and matter.
Notation of nuclear reactions:- Standard nuclear notation shows (see picture) the chemical symbol, the mass number and the atomic number of the isotope. If the initial nuclei are denoted by a and b, and the product nuclei are denoted by c and d, the reaction can be represented by the equation. a + b → c + d

Nuclear Fission:- There are nuclei that can undergo fission on their own spontaneously, but only certain nuclei, like uranium-235, uranium-233, and plutonium-239, can sustain a fission chain reaction. This is because these nuclei release neutrons when they break apart, and these neutrons can induce fission of other nuclei. Free neutrons released by each fission play a very important role as a trigger of the reaction.
Nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts (lighter nuclei). This nuclear reaction is triggered by the neutron.
Nuclear Fusion:- The process in which two nuclei of light elements (like that of hydrogen) combine to form a heavy nucleus (like that of helium), is called nuclear fusion. A tremendous amount of energy is produced during the fusion process. When two nuclei are brought together, they repel each other due to their similar charges.
Nuclear Fusion is a nuclear process, where the energy is generated by smashing together light atoms.
Nuclear Binding Energy:- Nuclei are made up of protons and neutrons, but the mass of a nucleus is always less than the sum of the individual masses of the protons and neutrons which constitute it. The difference is a measure of the nuclear binding energy which holds the nucleus together.
Nuclear power plant:- A nuclear power plant (nuclear power station) looks like a standard thermal power station with one exception. The heat source in the nuclear power plant is a nuclear reactor. As is typical in all conventional thermal power stations the heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity. The steam turbine is a common feature of all thermal power plants. Steam Turbine was invented in 1884 by Sir Charles Parsons, whose first model was connected to a dynamo that generated 7.5 kW (10 hp) of electricity. The exceptional feature of the nuclear power plant is the nuclear reactor and its safety and auxiliary systems.
As you can see the nuclear power plant consist of two main buildings:
Containment building (houses Nuclear Reactor) and Turbine building (houses Turbo Generator)








Comments